Prescribing medication by its active ingredient will become mandatory from February 2021. This change is part of the government’s 2018-2019 Electronic Prescribing Budget initiative that was legislated in 2019 under the National Health (Pharmaceutical Benefits) Amendment (Active Ingredient Prescribing) Regulations 2019. The aim of this regulation is to improve patient understanding of the medications they take in addition to promoting the uptake of generic and biosimilar medicines that would support a long term viable and sustainable market for these medications in Australia.
The regulation mandates the inclusion of the active ingredients on all PBS and RPBS prescriptions with the exception of:
- Handwritten prescriptions
- Paper-based medication charts in residential aged care settings
- Medications with four or more active ingredients
- Vaccines
- Custom preparations and prescriptions generated through a free text function within prescribing software
- Over the counter items
- Non-medicinal items such as dressings and food supplements
- Medications determined by the Secretary for practicality and safety reasons
Brand names can be included in the prescription if it is considered to be clinically necessary by the prescriber; however, the active ingredient must be listed first as per the regulation. Furthermore, software is prohibited from automatically including the brand name by default. It is therefore up to the prescriber to include the brand name on the prescription.
Why is this change being implemented?
There are numerous benefits to prescribing by active ingredient rather than brand name. First, generic prescribing enables patients to identify the pharmaceutically active ingredient (the international nonproprietary name) of their drug and thereby have a better understanding about the medications they take.
Second, it will reduce the risk of patients accidentally taking the same medication as a result of a prescribing or dispensing error due to being unaware that the brand name is not a unique identifier of their medication.
Third, it will allow the dispensation of any suitable equivalent generic should their brand of medication not be available at the pharmacy and subsequently reduce delays in supplying medication to the patient.
It is envisaged this change will increase the uptake of generic and biosimilar products which would reduce the out-of-pocket cost to the patient and the PBS.
How might this change the way I prescribe medications?
From a prescriber’s perspective, there are some changes in our workflow. When prescribing a new medication, we can still search by brand name or the active ingredient.
Prescribing a new medication by brand name
If we wish the patient to have a specific brand, then we have to check the “Print brand name on scripts” check-box and un-check the “Allow brand substitution” check box. This will convey to the pharmacist that the brand name on the script is what should be dispensed and brand substitution is not permitted.
Prescribing a new medication by active ingredient name
If we are satisfied that there is no clinical need for the patient to be on a particular brand of medication then we can search and select the drug by the active ingredient name. The options to “Print brand name on scripts” and “Allow brand substitution” will not be selectable as it is superfluous information since we have chosen to prescribe a generic medication.
Providing a prescription for a patient’s existing medication by brand
During the roll out of the software update for active ingredient prescribing, if a patient’s medication has previously been declared as not allowing brand substitution, then it will be set to “Print brand name on scripts”. This is because a prescriber has previously decided and recorded that the patient must be on the recorded brand of medication. In such cases the brand name will be printed on the script and the workflow for the doctor will not change.
However, if “Allow Brand Substitution” is checked (meaning that a generic brand can be dispensed), then the “Print brand name on scripts” will not be flagged. This is because, it has not previously been declared that the patient must be on that brand of medication. As per the regulation, software vendors cannot default to printing brand name on scripts in such cases. This may potentially become an issue to doctors who have, for example, previously prescribed “Micardis”, but have declared that brand substitution is permitted. In these cases, the brand “Micardis” will not be printed on the script and therefore the patient will be dispensed a generic Telmisartan rather than Micardis.
Providing a prescription for a patient’s existing medication by active ingredient
This scenario should not change the workflow of the doctor as the active ingredient will be printed.
How might this change affect my patients?
It is very important that we have a discussion with our patients regarding the upcoming changes and how it can affect their medications. This is especially if they are taking brand medications and we wish them to continue to do so.
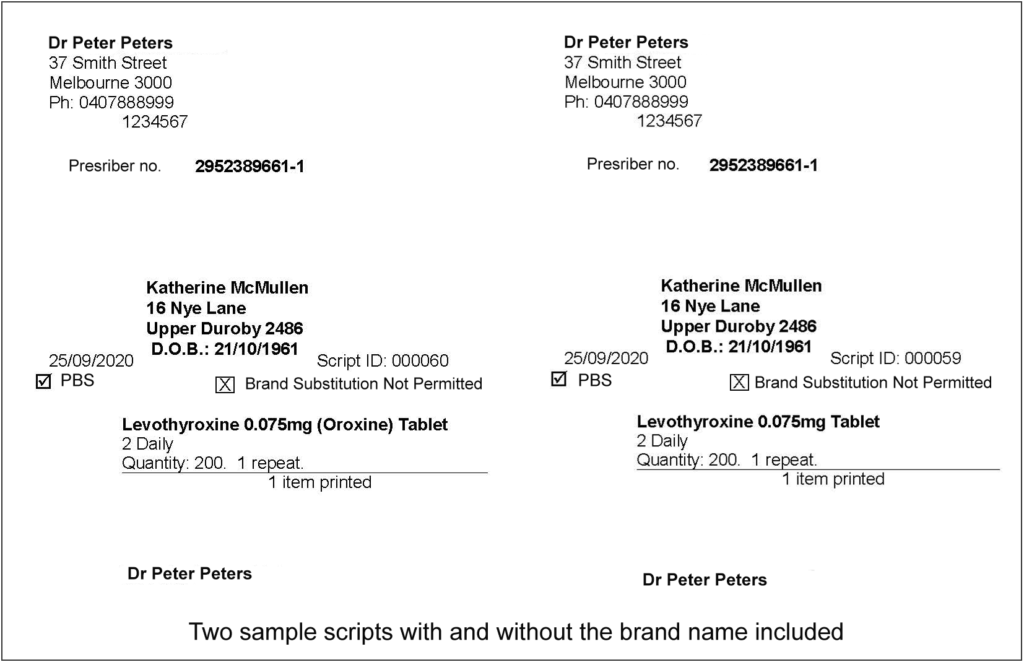
Below are some examples of a typical prescription showing branded medication, and the same prescription showing only the active ingredient.
Authored by:
Dr Fabrina Hossain
Clinical Advisor at Best Practice Software
To keep up to date with Active Ingredient Prescribing, and to be notified when further information and training materials are available, please sign up to our Educate Newsletter.

